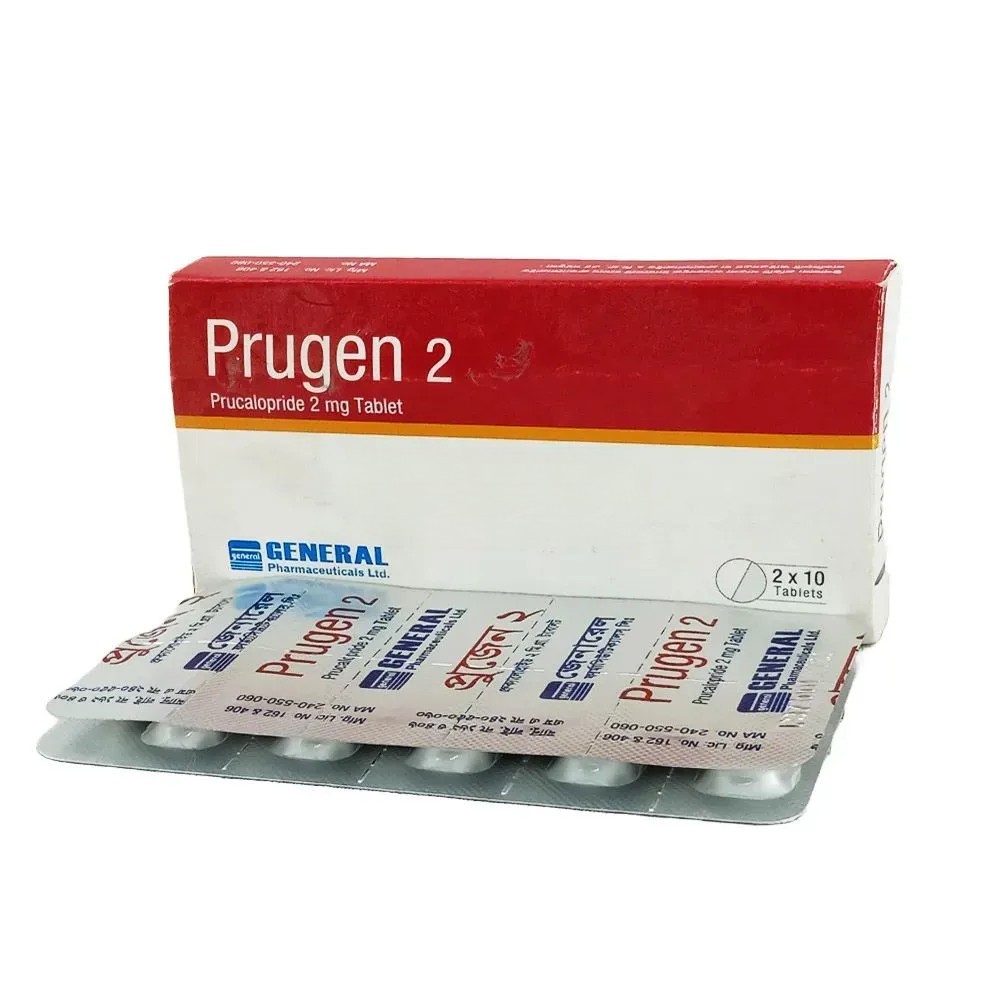

Prugen Tablet, Prucalopride Succinate 2 mg
Inhouse product
-
৳11.40
৳12.00 -
৳42.75
৳45.00 -
৳16.63
৳17.50 -
৳2.14
৳2.25
Reviews & Ratings
Indications
Prugen tablet is
indicated for symptomatic treatment of chronic constipation in adults in whom
laxatives fail to provide adequate relief.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Pharmacology
Prucalopride acts as a
selective stimulator of the 5-HT4 receptors while having no interaction with
hERG channel or 5-HT1 receptors which reduces significantly the cardiovascular
risk found in other similar drugs. 5-HT4 receptors can be found throughout the
gastrointestinal tract primarily in smooth muscle cells, enterochromaffin
cells, and myenteric plexus. Its activation produces the release of
acetylcholine which is the major excitatory neurotransmitter in the GI tract.
Hence, prucalopride stimulates motility by interacting specifically with 5-HT4
receptors in the GI tract which causes a release of acetylcholine and further
contraction of the muscle layer of the colon and relaxation of the circular
muscle layer leading to the propulsion of luminal content.
Dosage &
Administration
Adults: 2 mg once daily with or without food, at any
time of the day. Due to the specific mode of action of prucalopride
(stimulation of propulsive motility), exceeding the daily dose of 2 mg is not
expected to increase efficacy.
Older people: Start with 1 mg once daily; if needed the
dose can be increased to 2 mg once daily.
Children: Prucalopride should not be used in children
and adolescents younger than 18 years
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Interaction
In-vitro data indicate
that, Prugen has a low interaction potential and therapeutic concentrations of
Prugen are not expected to affect the CYP-mediated metabolism of co medicated
medicinal products. Although Prugen may be a weak substrate for P-glycoprotein
(P-gp), it is not an inhibitor of P-gp at clinically relevant concentrations.
Ketoconazole (200 mg b.i.d.), a potent inhibitor of CYP3A4 and of P-gp,
increased the systemic exposure to prucalopride by approximately 40%. This
effect is too small to be clinically relevant. Interactions of similar
magnitude may be expected with other potent inhibitors of P-gp such as
verapamil, cyclosporine A and quinidine.
Studies in healthy subjects showed that, there were no clinically relevant
effects of Prugen on the pharmacokinetics of warfarin, digoxin, alcohol,
paroxetine or oral contraceptives.
Contraindications
Prucalopride is
contraindicated in those people who are hypersensitive to the active substance
or to any of the excipients and people with renal impairment requiring
dialysis.
Side Effects
The most frequently
reported adverse reactions associated with Prugen therapy are headache (17.8%)
and gastrointestinal symptoms (abdominal pain), nausea and diarrhoea. The
adverse reactions occur predominantly at the start of therapy and usually
disappear within a few days with continued treatment. Other adverse reactions
have been reported occasionally. The majority of adverse events were mild to
moderate in intensity.
Pregnancy &
Lactation
Prucalopride is not
recommended during pregnancy and women of childbearing potential should use
effective contraception during treatment. Animal studies do not indicate direct
or indirect harmful effects with respect to pregnancy, embryonal/fetal
development, parturition, or postnatal development. In the absence of human
data, it is not recommended to use Prucalopride during breastfeeding
Precautions &
Warnings
- Renal excretion is the main
route of elimination of prucalopride. A dose of 1 mg is recommended in
subjects with severe renal impairment.
- Caution should be exercised
when prescribing Prugen to patients with severe hepatic impairment
(Child-Pugh class C) due to limited data in patients with severe hepatic
impairment.
- In case of severe diarrhoea,
the efficacy of oral contraceptives may be reduced and the use of an additional
contraceptive method is recommended to prevent possible failure of oral
contraception.
- The tablets contain lactose.
Patients with rare hereditary problems of galactose intolerance, the Lapp
lactase deficiency or glucose-galactose malabsorption should not take this
medicinal product.
Use in Special
Populations
Renal Impairment: The dose for patients with severe renal
impairment (GFR <30 ml/min/1.73 m2) is 1 mg once daily. No dose
adjustment is required for patients with mild to moderate renal impairment.
Hepatic Impairment: Patients with severe hepatic impairment
(Child-Pugh class C) start with 1 mg once daily which may be increased to 2 mg
if required to improve efficacy and if the 1 mg dose is well tolerated. No dose
adjustment is required for patients with mild to moderate hepatic impairment.
Overdose Effects
An overdose may result
in symptoms resulting from an exaggeration of prucalopride's known
pharmacodynamic effects and include headache, nausea and diarrhoea. Specific
treatment is not available for Prugen overdose. Should an overdose occur, the
patient should be treated symptomatically and supportive measures instituted,
as required. Extensive fluid loss by diarrhoea or vomiting may require
correction of electrolyte disturbances.
Therapeutic Class
Osmotic purgatives
Storage Conditions
Store at room
temperature, below 30°C. Do not remove desiccant. Dispense in original bottle.
Frequently Bought Products
Ace Suppository250 mgParacetamol
Siglita Tablet, Sitagliptin 50 mg
Methipred Tablet, Methylprednisolone 4 mg
Amlopin Tablet, Amlodipine Besilate 10 mg
Eclo Cream 10 gm tube, Clobetasol Propionate 0.05%
Bet-A Cream 20 gm tube, Betamethasone Valerate 0.1%
Product Queries (0)
Login Or Registerto submit your questions to seller
Other Questions
No none asked to seller yet
-
৳11.40
৳12.00 -
৳42.75
৳45.00 -
৳16.63
৳17.50 -
৳2.14
৳2.25